Introduction
In recent years, the treatment of B-cell acute lymphoblastic leukemia (B-ALL) has evolved tremendously resulting in improved outcomes in patients with relapse/refractory (r/r) B-ALL and patients with measurable residual disease (MRD+). One such novel therapy is the CD3/CD19 bispecific antibody blinatumomab. There is however limited data on the outcomes and treatment options for B-ALL patients who relapse after blinatumomab was given as salvage therapy.
Methods
This IRB-approved study retrospectively reviewed the outcomes of all adult patients (≥18 years old) with B-ALL treated with blinatumomab at our center, Singapore General Hospital. The primary aim was to describe the incidence, risk factors and survival outcomes of B-ALL patients who relapse after blinatumomab salvage therapy.
Results
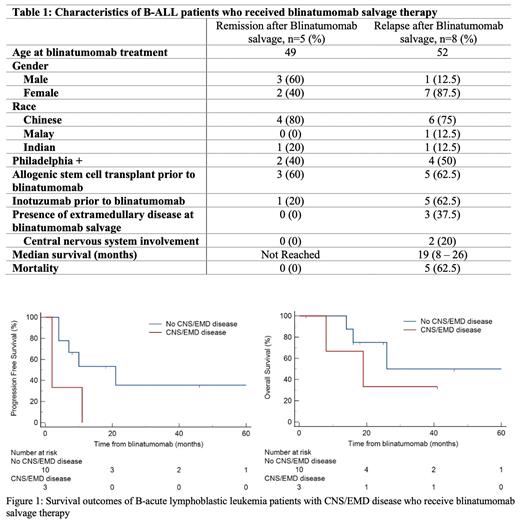
Between January 2017 to December 2022, a total of 27 patients were treated at our center with blinatumomab. Blinatumomab was used as salvage therapy in 13 patients and for MRD+ disease after front-line therapy in 7 patients. Characteristics of the 13 patients who received blinatumomab salvage therapy are shown in Table 1. Eight patients had received an allogenic hematopoietic stem cell transplant (alloHSCT) and 6 patients had received inotuzumab, a CD22 antibody-drug conjugate, prior to blinatumomab salvage. The median number of lines of therapy before blinatumomab salvage was 3 and the median number of cycles of blinatumomab administered was 4. All but 1 patient achieved MRD negativity after the prescribed number of blinatumomab cycles. Eight (61.5%) of 13 patients relapsed after blinatumomab salvage therapy. Median progression free survival (PFS) in this group of patients was 7 months (IQR 2-11) and overall survival (OS) of 19 months (IQR 8-26). Six (75%) of these 8 patients proceeded to receive salvage therapy with inotuzumab. Majority (5 of 6) achieved MRD negativity and 2 patients subsequently underwent alloHSCT and CAR-T therapy respectively. Unfortunately, all 6 patients eventually relapsed. Of note, 3 patients had received inotuzumab prior to blinatumomab salvage.
We observed that patients (n=3) with extramedullary disease (EMD) (including central nervous system (CNS) involvement) at initiation of blinatumomab salvage therapy were associated with higher odds of both systemic and extramedullary relapse (p=0.014). These patients had inferior progression free survival (PFS) as compared to patients without EMD/CNS involvement, median PFS 2 months (95% CI 2 - 11) vs 21 months (95% CI 4 - 21), p = 0.041 (Figure 1). A trend towards inferior overall survival was also observed though statistical significance was not demonstrated, median OS 19 months (95% CI 8 - 19) vs 26 months (95% CI 14 - 26).
Conclusion
EMD/CNS involvement prior to blinatumomab salvage therapy was associated with higher risk for systemic and EMD/CNS relapse. Patients with B-ALL who relapsed after blinatumomab salvage could be successfully rescued by inotuzumab regardless of prior inotuzumab use. These groups of patients however represent very-high risk groups with inferior survival outcomes. Novel treatments and better sequencing of treatments may improve outcomes of these patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal